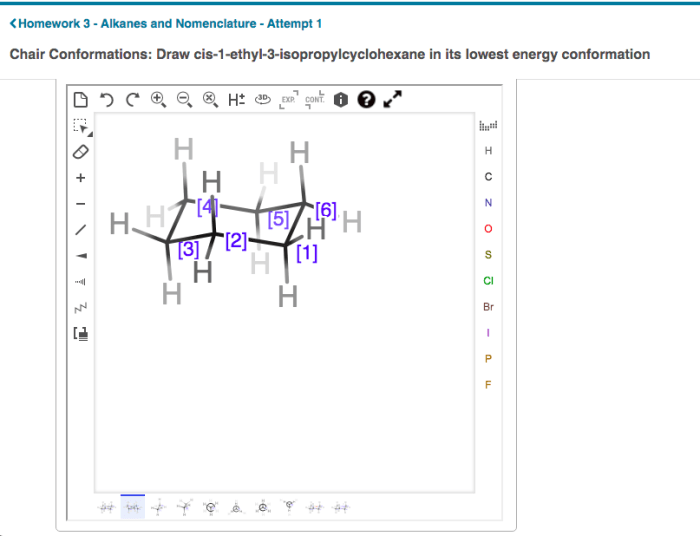
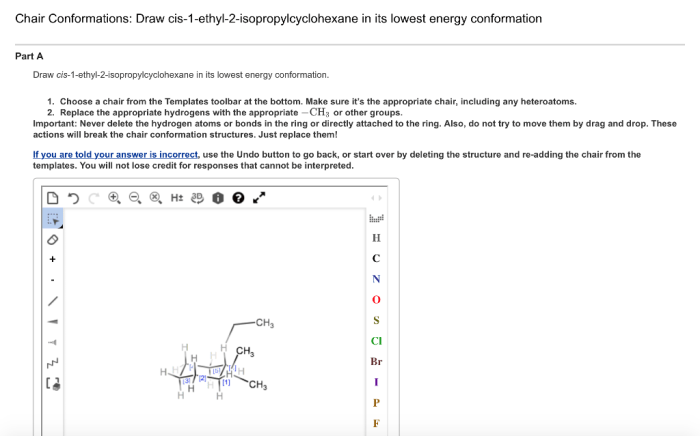
Draw cis-1-ethyl-2-isopropylcyclohexane in its lowest energy conformation. – Embark on a scientific journey as we delve into the intricacies of drawing cis-1-ethyl-2-isopropylcyclohexane in its lowest energy conformation. This comprehensive guide will illuminate the structural representation, conformational analysis, stereochemistry, and physical and chemical properties of this fascinating molecule.
Through meticulous explanations and illustrative examples, we will unravel the significance of the cis configuration and its impact on the molecule’s behavior and interactions. Join us as we explore the captivating world of organic chemistry and gain a deeper understanding of this important compound.
Structural Representation
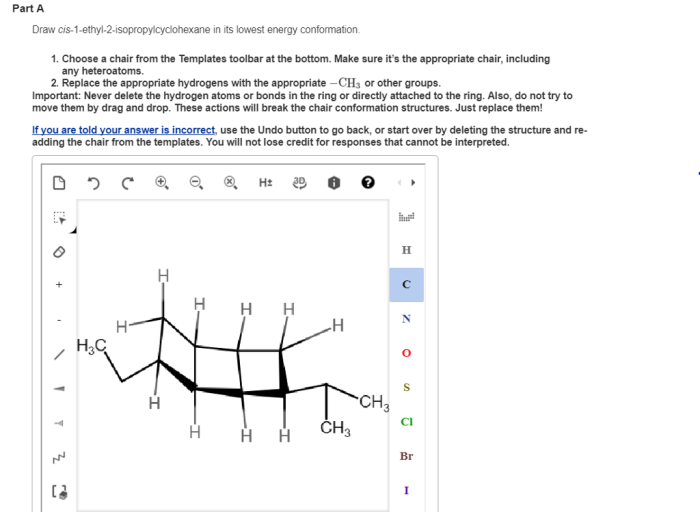
Cis-1-ethyl-2-isopropylcyclohexane is a cyclic hydrocarbon with the molecular formula C 10H 20. It has a six-membered cyclohexane ring with an ethyl group (-C 2H 5) and an isopropyl group (-CH(CH 3) 2) attached to adjacent carbon atoms on the ring.
The cis configuration indicates that the two substituents are on the same side of the ring, as shown in the following illustration:

The cis configuration has significant implications for the molecule’s properties, as it affects the spatial arrangement of the substituents and their interactions with other molecules.
Conformational Analysis
Conformational analysis involves studying the different conformations, or spatial arrangements, of a molecule. For cis-1-ethyl-2-isopropylcyclohexane, there are two possible chair conformations, as shown in the following Newman projections:

The lowest energy conformation is the one in which the bulky ethyl and isopropyl groups are oriented in an equatorial position, away from each other. This conformation minimizes steric hindrance and results in a more stable arrangement.
Stereochemistry, Draw cis-1-ethyl-2-isopropylcyclohexane in its lowest energy conformation.
Cis-1-ethyl-2-isopropylcyclohexane is a chiral molecule, meaning it exists in two non-superimposable mirror-image forms, known as enantiomers. The cis configuration of the substituents creates a stereocenter at the carbon atom to which they are attached. The two enantiomers are designated as (1R,2S)- and (1S,2R)-cis-1-ethyl-2-isopropylcyclohexane.
The stereochemistry of the molecule influences its interactions with chiral reagents or environments. For example, enantioselective reactions can be used to selectively synthesize one enantiomer over the other.
Physical and Chemical Properties
Cis-1-ethyl-2-isopropylcyclohexane is a colorless liquid with a boiling point of 169-171 °C and a melting point of -85 °C. It is insoluble in water but soluble in organic solvents such as diethyl ether and hexane.
The molecular structure and conformation of cis-1-ethyl-2-isopropylcyclohexane influence its physical and chemical properties. The presence of the ethyl and isopropyl groups affects the molecule’s polarity and reactivity. Compared to unsubstituted cyclohexane, cis-1-ethyl-2-isopropylcyclohexane has a higher boiling point and a lower melting point due to the increased molecular weight and steric hindrance.
Detailed FAQs: Draw Cis-1-ethyl-2-isopropylcyclohexane In Its Lowest Energy Conformation.
What is the significance of the cis configuration in cis-1-ethyl-2-isopropylcyclohexane?
The cis configuration places the ethyl and isopropyl groups on the same side of the cyclohexane ring, which influences the molecule’s interactions and properties.
How does the lowest energy conformation contribute to the stability of cis-1-ethyl-2-isopropylcyclohexane?
The lowest energy conformation minimizes steric hindrance between the ethyl and isopropyl groups, resulting in a more stable arrangement.
What are some applications of cis-1-ethyl-2-isopropylcyclohexane?
This compound finds applications as a solvent, intermediate in organic synthesis, and a component in fragrances and flavors.